Kalkine has a fully transformed New Avatar.

BioCryst Pharmaceuticals, Inc.
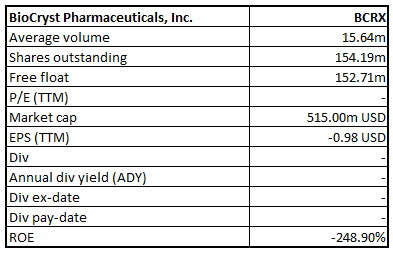
BCRX Details
BCRX Commences Study on Galidesivir for COVID-19 Virus: BioCryst Pharmaceuticals, Inc. (NASDAQ: BCRX) is one of the leaders in the use of crystallography and structure-based drug design for the advancement of innovative therapeutics to cure cancer, cardiovascular diseases, autoimmune diseases, and viral infections. On 9 April 2020, BCRX stated that it has begun registration in a clinical study to gauge the wellbeing, safety and clinical as well as antiviral effects of Galidesivir in patients with COVID-19 disease. The trial was financed by the National Institute of Allergy and Infectious Diseases (NIAID). Notably, the company is set to report its 1QFY20 results on 6 May 2020.
Other Recent Update: In another update, the company stated that it has appointed Anthony Doyle as its new senior vice president and chief financial officer (CFO).
4QFY19 Key Highlights for the Period Ended 31 December 2019: During the quarter, the company reported total revenues of $39.7 million, up from $2.7 million in the year-ago period. The increase was due to $20.1 million upfront payment from Torii to curb the HAE attacks, and $13.9 million of RAPIVAB® product sales. Research and development (R&D) expenses stood at $26.8 million, up from $23.4 million reported in the year-ago period, on the back of higher spending and other preclinical development initiatives. Net loss for the quarter stood at $2.6 million, as compared to a net loss of $27.4 million, reported in the year-ago period. Selling, general and administrative (SG&A) expenses increased from $4.5 million reported in the year-ago period to $10.5 million, due to higher spending.

Revenues Highlight (Source: Company Reports)
Balance Sheet Position: At the end of 31 December 2019, cashand investments stood at $137.8 million, indicating a rise from $128.4 million at the end of December 31, 2018. Total assets at the end of the period amounted to $175.3 million. During the quarter, cash used in operations amounted to $33.5 million.
Outlook for FY20: For FY20, the company expects net cash used in operating activities to be between $125 to $150 million. BCRX further expects operating expenses to be between $135 to $160 million, which eliminates equity-based compensation expense. Further, the company expects approval and launch of oral, once daily berotralstat in Japan in the second half of 2020. The company is receiving positive response from both HAE patients and physicians about the accessibility of oral medicines to handle and treat their disease.
Stock Recommendation: The stock of BCRX closed at $3.34 with a market capitalization of $515 million. The stock made a 52-week low and high of $1.38 and $8.15 and is currently trading at the lower band of the range. The stock of the company has run up 12.46% in the last three months and 74.87% in the last one month.The companyhas witnessed a substantial rise in its share price in the last couple of months, due to its ongoing efforts associated with the clinical trialsto curb theCOVID-19 impact.Notably, the company’s debt to equity ratio stood at 2.09x in FY19, higher than the D/E ratio of 1.26x in FY18. On the valuation front, the stock is trading at an EV/Sales multiple of 10.4x as compared to the industry median of 6x on TTM (Trailing Twelve Months) basis. Considering the latest results, valuation on TTM basis, and current trading levels, we have a watch stance on the stock at the closing price of $3.34, down 5.92% as on 28 April 2020.
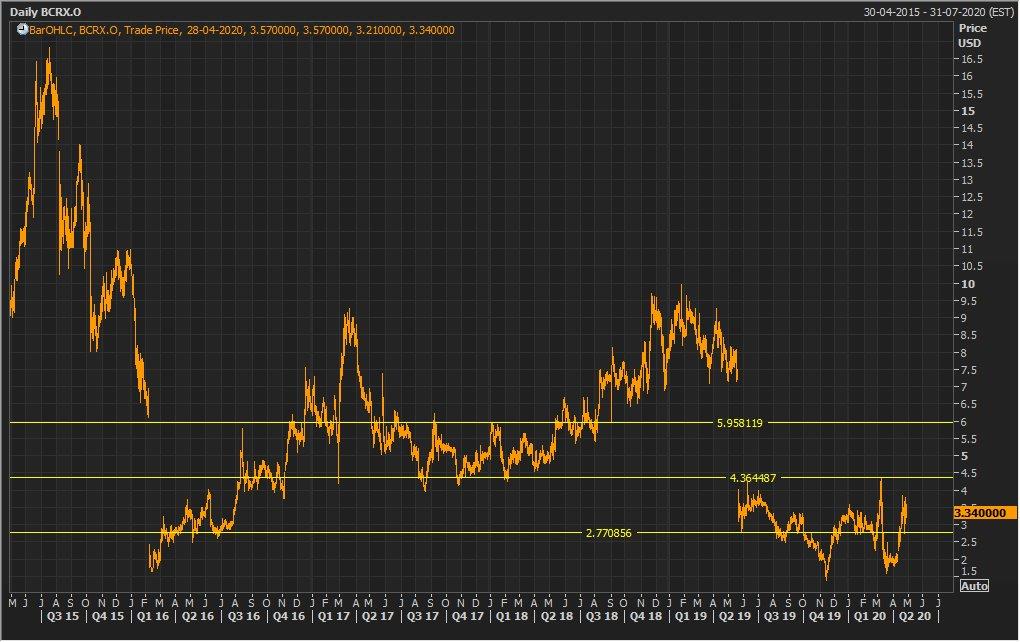
BCRX Daily Technical Chart (Source: Thomson Reuters)
Disclaimer
The advice given by Kalkine Pty Ltd and provided on this website is general information only and it does not take into account your investment objectives, financial situation or needs. You should therefore consider whether the advice is appropriate to your investment objectives, financial situation and needs before acting upon it. You should seek advice from a financial adviser, stockbroker or other professional (including taxation and legal advice) as necessary before acting on any advice. Not all investments are appropriate for all people. Kalkine.com.au and associated pages are published by Kalkine Pty Ltd ABN 34 154 808 312 (Australian Financial Services License Number 425376). The information on this website has been prepared from a wide variety of sources, which Kalkine Pty Ltd, to the best of its knowledge and belief, considers accurate. You should make your own enquiries about any investments and we strongly suggest you seek advice before acting upon any recommendation. Kalkine Pty Ltd has made every effort to ensure the reliability of information contained in its newsletters and websites. All information represents our views at the date of publication and may change without notice. To the extent permitted by law, Kalkine Pty Ltd excludes all liability for any loss or damage arising from the use of this website and any information published (including any indirect or consequential loss, any data loss or data corruption). If the law prohibits this exclusion, Kalkine Pty Ltd hereby limits its liability, to the extent permitted by law to the resupply of services. There may be a product disclosure statement or other offer document for the securities and financial products we write about in Kalkine Reports. You should obtain a copy of the product disclosure statement or offer document before making any decision about whether to acquire the security or product. The link to our Terms & Conditions has been provided please go through them and also have a read of the Financial Services Guide. On the date of publishing this report (mentioned on the website), employees and/or associates of Kalkine Pty Ltd do not hold positions in any of the stocks covered on the website. These stocks can change any time and readers of the reports should not consider these stocks as personalised advice.