Kalkine has a fully transformed New Avatar.

.png)
Stocks’ Details
Ramsay Health Care Limited
Revenues up ~22.5% Year Over Year: Ramsay Health Care Limited (ASX: RHC) is engaged in providing numerous healthcare facilities in Australia, France, Indonesia, Malaysia, and the United Kingdom. The market capitalisation of the company stood at $14.91 Bn as on 25th May 2020. Recently, the company stated that it has successfully raised $300 million, under its Share Purchase Plan (SPP), which was announced on 29 April 2020.
RHC Finalises Agreements: The company recently announced that it has finalised the comprehensive agreement with NHS England, under which it will provide its facilities and services during the COVID-19 pandemic.As per the deal, the agreement is for a minimum of 14 weeks, starting from 23 March 2020.In another update, the company also finalised the agreement with the State of Western Australia (the State) and State of Queenslandto provide same facility during the COVID19 pandemic.Previously, the company has also finalised a similar agreement with the State of Victoria. Revenues during 1HFY20 went up 22.5% year over year and came in at $6,332.5 million.
.png)
Key Highlights (Source: Company Reports)
Withdrawal of Guidance: Considering the continuing high level of uncertainty surrounding the spread, duration and impact of coronavirus, the company has withdrawn its earnings guidance for FY20.
Valuation Methodology:Price to Earnings Multiple Based Relative Valuation (Illustrative).png)
Price to Earnings Multiple Based Valuation (Source: Refinitiv, Thomson Reuters)
Note: All forecasted figures and peers have been taken from Thomson Reuters, NTM-Next Twelve Months
Stock Recommendation: As per ASX, the stock of RHC gave a return of 10.21% in the last one month. The stock is trading above the mid-point of its 52-week high and low level of $80.93 and 46.13, respectively.The company is currently undertaking decisive actions to strengthen its balance sheet, so that it can navigate through the current uncertain operating environment. Net margin of the company stood at 4.2% in 1H FY20, which is higher than the industry median of 3.0%. We have valued the stock using the P/E multiple based illustrative relative valuation method and arrived at a target price with an upside of lower double-digit (in percentage terms). For the purpose, we have taken peers like Cochlear Ltd (ASX: COH), Sonic Healthcare Ltd (ASX: SHL) and Resmed Inc (ASX: RMD). Therefore, in light of the improvement in net margin, current trading level, and recent equity raising amid COVID-19, we give a “Hold” recommendation on the stock at the current market price of $69 per share, up by 3.448% on 25 May 2020.
Atomo Diagnostics Limited
Revenues Increased Year Over year: Atomo Diagnostics Limited (ASX: AT1) is engaged in the development of global sales and continued expansion of its rapid test device technologies. As on 25 May 2020, the market capitalization of the company stood at ~$201.99 million. In a recent update, the company stated that Walker Group Holdings Pty Limited, a substantial holder of the company, has decreased its voting power from 10.39% to 8.32%.
Additional Order for COVID-19 Test Devices: The company recently stated that it has achieved an additional unscheduled purchase order from NG Biotech, SAS to supply 422,000 integrated blood test devices for the rapid blood based testing of antibodies produced in response to COVID-19 pandemic. The additional order, which was received between late March and mid-April, totals to 947,200 devices.
1HFY20 Key Highlights: During the half year ended 31 December 2019, the company recorded a significant increase in sales to $937k, up from $64k in 1HFY19. This increase was on the heels of acceleration of registrations and in-country rollout of HIV products by the AT1 HIV products distribution partners, along with the launch in Europe of customer RDT products that utilize the Pascal device. The company has total borrowings of $23.9 million as of 31 December 2019. Cash and cash equivalents at the end of 31 December 2019 stood at $12.4 million.
The company raised approximately $30 million from its IPO. Notably, the company intends to utilize proceeds of the IPO for expansion, R&D, and to repay its existing debt. The remaining amount will be used for working capital purposes. We believe that the raised fund will deleverage its balance sheet and will create long-term value for shareholders. Further, it will increase the company’s financial strength and will make it well-poised to stay afloat in the current uncertain scenario.
.png)
Growth in Revenue (Source: Company Reports)
Growth Prospects: The company is witnessing accelerated growth with a broad range of rapid test opportunities. It is focusing on commercializing COVID-19 rapid antibody tests globally and has signed agreements.
Risk Analysis: The main risks of the company include liquidity risk and market risk comprising interest rate risk and foreign currency fluctuation risk. Further, stiff competition from peers likes Abingdon Health, BioFire Diagnostics, and Proxim Diagnostics, to name few, remains a potential headwind.
Stock Recommendation: As per ASX, the stock of AT1 is trading close to its 52-weeks’ low level of $0.320, proffering a decent opportunity to enter the market. The stock of AT1 corrected by ~14.29% in the last one month. The company has a robust IP protection and scalable production with a cost-effective supply chain capability. AT1 has a proven technology for blood-based rapid test devices and has established a strong market position with sales of over 1.2 million devices. During 1H20, gross margin of the company witnessed a substantial improvement over the previous half and stood at 42%, up from 16.3% in 2H19. Considering the trading levels, strengthened foothold in the market, and increasing production orders, we recommend a ‘Speculative Buy’ rating on the stock at the current market price of $0.325, down by 9.722% on 25 May 2020.
Invex Therapeutics Ltd
Results of Phase 2 & 3 Clinical Trials: Invex Therapeutics Ltd (ASX: IXC) is a biopharmaceutical company, which is engaged in the treatment of neurological conditions through its Exenatide drug. As on 25 May 2020, the market capitalization of the company stood at $91.58 million. The company targets the orphan disease Idiopathic Intracranial Hypertension (IIH) and expects top-line Phase III data in second half of FY2023, along with obtaining regulatory approval for Presendin™ in 2024. Further, the company opines that it strongly supports moving Presendin™ into Phase III clinical development in 1HFY21. The data signifies statistical noteworthy reduction in Intracranial Pressure (ICP) shown in IIH patients receiving Exenatide drug at 2.5 hrs, 24 hrs and 12 weeks. This efficiency in primary and secondary endpoints validates a strong and sustained drug effect in the IIH population.
Successful Share Replacement: On 22 May 2020, the company announced a $26.2 million successful share placement (Placement) to institutional, professional, and sophisticated Australian and overseas investors. The amount raised from the Placement will be utilized to fully fund the planned Phase III Presendin™ registration clinical trial for Idiopathic Intracranial Hypertension (IIH).
March 2020 Quarter Update: At the end of the quarter ended 31 March 2020, the company had a cash balance of $10.4 million. Net cash used in operating activities for the quarter ended 31 March 2020, came in at $416K, after making major payments for staff costs, administration and corporate costs, along with R&D expenditure of $21K, $134K and $300K, respectively.
.png)
Cash Flow Details (Source: Company Reports)
Stock Recommendation: As per ASX, the stock of IXC is trading above the average of its 52-week low and high of $0.520 and $2.050, respectively. The stock of IXC went up by ~64.85% in the last one month.On the valuation front, the stock is trading at a P/BV multiple of 5.4x as compared to the industry median of 2.6x on TTM (Trailing Twelve Months) basis. Considering the aforesaid facts, we have a watch stance on the stock at the current market price of $1.49, down by 10.511% on 25 May 2020.
Noxopharm Limited
NOX Lodged pre-IND submission for a clinical trial of Veyonda: Noxopharm Limited (ASX: NOX) is involved in drug development, with key focus on the clinical development of Veyonda®. The market capitalisation of the company stood at $34.26 Mn as on 25 May 2020. In a recent update, the company informed that it has despatched the relevant documents in relation to its 1 for 2.5 non-renounceable pro rata entitlement offer on 20th May 2020. The issue relates to a capital raising of approximately $7.9 million, aimed at funding significant commercial opportunities that lie ahead. Recently, the company stated that it has requested for a pre-Investigational New Drug (pre-IND) plan with FDA for clinical testing of Veyonda® in patients, suffering from COVID-19 infection. The positive evaluation by FDA of the pre-IND, will pave way for a new option submitted to high importance COVID-proposals and will substantially reduce the time and complication of the FDA review process.
Other Recent Update: During the March 2020 quarter, the company made strong progress in its core business of developing Veyonda® as a new treatment for end-stage prostate cancer and in the development of a drug pipeline. In addition, the company has also received approval for first IND application for Veyonda® by US-FDA in February 2020. During the same quarter, the Australian Patent Office allowed the Australian patent application for Veyonda®.
March 2020 Quarter Update: At the end of the quarter ended 31 March 2020, the company had a cash balance of ~$2.03 million. Net cash used in operating activities for the quarter ended 31 March 2020, came in at $3.1 million, after making major payments for staff costs, administration and corporate costs along with R&D expenditure of $676K, $541K and $1.8 million, respectively.
.png)
Cash Flows (Source: Company Reports)
DAART-2 Trial Update: Following the successful completion of the DARRT-1 trial in December 2019, the company has continued to advance its planning and preparations for the DAART-2 trial during March 2020 quarter. The DARRT-2 trial is anticipated to begin in early 2021.
Stock Recommendation: The stock of the company gained 41.55% in the last one month and is currently trading below the average of its 52-week trading range of $0.084 - $0.618. On the valuation front, the stock is trading at an EV/Sales multiple of 11.9x, against the industry median of 9.6x on TTM basis. As on 31st December 2019, the company had cash and total borrowings amounting to $1.65 million and $7.46 million, respectively. Considering the ongoing clinical trials, pending request with US-FDA for a pre-Investigational New Drug (pre-IND) plan, recent capital raising announcement, amount of cash and borrowings as at 31st December 2019, and valuation, we have a wait and watch stance on the stock at the current market price of $0.225 as on 25th May 2020.
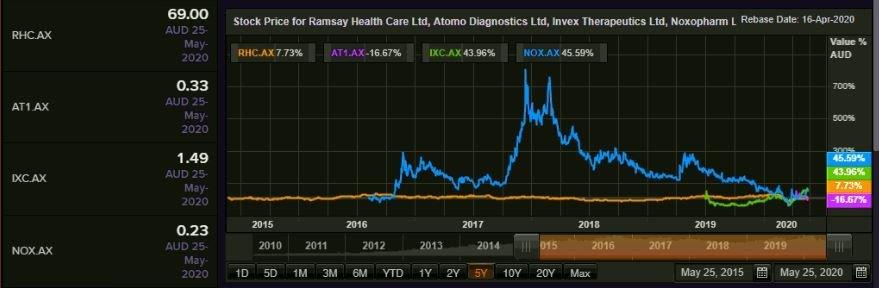
Comparative Price Chart (Source: Refinitiv, Thomson Reuters)
Disclaimer
The advice given by Kalkine Pty Ltd and provided on this website is general information only and it does not take into account your investment objectives, financial situation or needs. You should therefore consider whether the advice is appropriate to your investment objectives, financial situation and needs before acting upon it. You should seek advice from a financial adviser, stockbroker or other professional (including taxation and legal advice) as necessary before acting on any advice. Not all investments are appropriate for all people. Kalkine.com.au and associated pages are published by Kalkine Pty Ltd ABN 34 154 808 312 (Australian Financial Services License Number 425376). The information on this website has been prepared from a wide variety of sources, which Kalkine Pty Ltd, to the best of its knowledge and belief, considers accurate. You should make your own enquiries about any investments and we strongly suggest you seek advice before acting upon any recommendation. Kalkine Pty Ltd has made every effort to ensure the reliability of information contained in its newsletters and websites. All information represents our views at the date of publication and may change without notice. To the extent permitted by law, Kalkine Pty Ltd excludes all liability for any loss or damage arising from the use of this website and any information published (including any indirect or consequential loss, any data loss or data corruption). If the law prohibits this exclusion, Kalkine Pty Ltd hereby limits its liability, to the extent permitted by law to the resupply of services. There may be a product disclosure statement or other offer document for the securities and financial products we write about in Kalkine Reports. You should obtain a copy of the product disclosure statement or offer document before making any decision about whether to acquire the security or product. The link to our Terms & Conditions has been provided please go through them and also have a read of the Financial Services Guide. On the date of publishing this report (mentioned on the website), employees and/or associates of Kalkine Pty Ltd do not hold positions in any of the stocks covered on the website. These stocks can change any time and readers of the reports should not consider these stocks as personalised advice.