Company Overview: Clinuvel Pharmaceuticals Limited is a biopharmaceutical company. The Company is focused on developing drugs for the treatment of a range of severe skin disorders. Its lead compound, SCENESSE (afamelanotide 16 milligrams), prevents phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). The Company focuses at preventing the symptoms of skin diseases related to the exposure to harmful ultra violet (UV) radiation and at repigmentation of the skin due to a number of depigmentation disorders. SCENESSE has completed Phase II and III trials in the United States and Europe, and has been granted marketing authorization by the European Commission for adults with EPP. It has operations in Europe, the United States and Singapore. SCENESSE acts by increasing the levels of melanin in the skin and shields against UV radiation (UVR) and sunlight. SCENESSE is delivered via a subcutaneous dissolving implant.
.png)
CUV Details
Higher Investments, Strong Cash Position & CUV’s SCENESSE Drug are key Catalysts: Clinuvel Pharmaceuticals Limited (ASX: CUV) is an Australia based biopharmaceutical company, devoted to the development of drugs for the treatment of a range of severe skin illnesses. The company focuses on research and development initiatives for patient’s treatment by interaction of skin with its environments, seeking to provide innovative medical solutions for photoprotection, regimentation, and genetic defects. SCENESSE is CLINUVEL’s proprietary first-in-class photoprotective drug, which is used for the prevention of phototoxicity in adult patients with erythropoietic protoporphyria (EPP).
Total revenues for FY19 came in at approximately $31.05 million, depicting a rise of 22% from $25.49 million reported in FY18. The year over year increase can primarily be attributed to the company’s approach to supply SCENESSE to patients in Europe. Further, EPP expert centres in Europe continued to prescribe SCENESSE to higher number of new and existing patients for their skin treatment, which is a key positive. In FY19, the company’s net profit before tax came in at $18.11 million, representing a rise of 40% from $12.94 million reported in the prior corresponding period. Clinical demand during the year was pleasing, signifying the benefits earned out of investment for the development of SCENESSE. CUV declared a final dividend (fully franked) of 2 cents per share in FY19.
Looking at the past three-years performance over the period covering FY16 to FY19, the company witnessed a top-line CAGR of ~69.1%. Basic earnings per share came in at 37.6 cents per share, as compared to a loss of 7 cents per share reported in FY16. Profit attributable to shareholders over the same time span witnessed a CAGR of 79.8%, with FY16 and FY19 profit amounting to $3.12 million and $18.13 million, respectively.
Going forward, the company expects to advance its product pipeline, improve the expansion of the molecules CUV9900 and VLRX001 via formulation development, and non-clinical and human testing. The company has expanded its resources and its capabilities to progress these projects ongoing at VALLAURIX. Consequently, the company remains on track to achieve its long- term goal and maintain profitability. This in turn, will finance the company’s growth in the form of enhancement of products in the pipeline related to skin-related indicators and further strengthen its traction in the European market.
.png)
Sneak Peek at Shareholder Returns (Source: Company Reports)
FY19 Financial Highlights for the period ended 30 June 2019: In FY19, the company reported total revenue and other income amounting to $32.50 million, signifying a rise of 24% from $26.24 million reported in the year-ago period. Profit after tax came in at $18.13 million, up 37% on a year over year basis. During the period, earnings per share soared 36% year over year to 37.6 cents. The company’s commercial sales of SCENESSE in Europe stood at $26.49 million in FY19, up from $21.36 million from FY18. Unit sales for the period rose 20% on a yearly basis, indicating higher supply of SCENESSE to EPP Expert Centres across European countries. Reimbursement revenues under the Special Access Schemes for SCENESSE, increased 10% to $4.559 million for FY19. Moreover, the positive impact of foreign currency exchange rate, as a result of a stronger Euro relative to the Australian dollar was a key positive during the period.
.png)
FY19 Financial Performance (Source: Company Reports)
December Quarter 2019 Update: During the quarter ended 31st December 2019, cash receipts from customers amounted to $3.73 million, an increase of 43% on a year over year basis. For the 12 months ended 31st December 2019, receipts from customers increased 19% as compared to the prior corresponding period. At the end of the quarter, cash and cash equivalents stood at $57.44 million. Operating cash outflow for the quarter stood at $635K. For the coming quarter, the company expects cash outflow to be $5.02 million, after making major payments for staff costs and product manufacturing and operating costs of $2.2 million and $1.6 million, respectively.
.png)
Annual Recipes & Operating Payments(Source: Company Reports)
Balance Sheet & Cash Flow Position:The company exited FY19 with a cash balance of $54.27 million. Net assets of the company as at 30th June 2019 came in at $57.18 million, up from $39.42 million as at 1st July 2018, reflecting the impact of rise in revenue from commercial sales. The company did not raise any capital via equity or debt, representing a robust financial position for investing in future performance. Total dividends paid for FY19 amounted to $0.957 million.
Operating cash inflow for the year stood at $18.46 million, up from an operating cash inflow of $11.69 million in FY18. Net cash outflow from investing activities amounted to $0.257 million, and financing activities during the year led to an outflow of $1.03 million.
.png)
Cash Flow Statement (Source: Company Reports)
Recent Updates:
On 10th February 2019, the company announced that it has requested a meeting with the US Food and Drug Administration (FDA) relating to Type C Guidance meeting in order to avail approval on the design of a multicentre Phase IIb vitiligo clinical study (CUV104). Further, the meeting was requested to discuss on the necessary data package to support the filing for a supplemental New Drug Application CUV’s SCENESSE drug in vitiligo.
On 23rd December 2019, the company stated that it has applied for its SCENESSE drug to be registered in the Australian Register of Therapeutic Goods (ARTG). If the registration is accepted, then SCENESSE would be available in Australia for the prevention of phototoxicity in adult patients with erythropoietic protoporphyria (EPP).
Top 10 Shareholders: The top 10 shareholders have been highlighted in the table, which together form around 26.15% of the total shareholding. ACN 108 768 896 Pty. Ltd. held the maximum number of shares with a percentage holding of 9.16%, followed by Ender 1, L.L.C., with a holding of 5.24%.
.png)
Top Ten Shareholders (Source: Thomson Reuters)
Key Metrics: In FY19, the company had an EBITDA margin of 54.9%, up from the prior corresponding period margin of 48.5%. The margin has seen an improvement over the last three years period covering FY17 – FY19, with FY17 EBITDA margin at 41.6%. Operating margin also improved over the year with FY18 and FY19 margin at 48.4% and 54.6%, respectively. Net Margin of the company was reported at 57.4%, depicting an improvement on the prior corresponding period margin of 51.4%. The company had negligible debt during the year with a debt-to-equity ratio of 0.01x, as compared to the industry median of 0.15x, demonstrating a better financial position in comparison to peers.
.png)
Key Metrics (Source: Thomson Reuters)
Outlook: The Company remains on track to execute its expansion plan in the USA, following the approval by the US Food & Drug Administration (FDA) to market SCENESSE for the treatment of adult EPP patients. Going forward, the company anticipates that its European business is likely to generate healthy earnings to finance its growth by boosting skin-related products in the pipeline. This in turn will strengthen its foothold in the European distribution market.
It is worth mentioning that the company stands to benefit from encouraging trends, which includes new drug approvals, an accelerated pace of innovation, promising drug launches, growing importance of biosimilars, cost-cutting initiatives, an aging population, expanding insurance coverage, the rising middle-class, insatiable demand for new drug, and an ever-increasing health care spending. The company aims to spend more on research and development to understand human biology and develop the world’s first photoprotective drug, CLINUVEL.
.png)
Key Valuation Metrics (Source: Thomson Reuters)
Valuation Methodology: P/E Market-Multiple Approach
.png)
P/E Market-Multiple Approach (Source: Thomson Reuters)
Note: All forecasted figures have been taken from Thomson Reuters
Stock Recommendation: The stock of the company generated positive returns of 13.92% in the past one year. In FY19, the company delivered a robust result, on the back of higher number of SCENESSE units supplied to patients in Europe. Further, the positive impact of foreign currency exchange rate exchange is a key positive. At CMP of $25.650, the stock of the company is trading at a P/E multiple 69.2x with a dividend yield of 0.1%. From the analysis standpoint, the company has recorded revenue CAGR of 69.1% over the last three years. RoE stood at ~37.5% in FY19, as compared to the industry median of -10%.
The company has a market capitalisation of ~$1.29 billion and ~49.41 million outstanding shares. Currently, the stock is trading below the average of its 52-week high and low of $45.88 and $21.00, respectively, proffering an opportunity for share accumulation. Considering the recent developments with respect to the key product SCENESSE®, strong cash position, and decent outlook, we have valued the stock using a 3-year average P/E market multiple of ~45.40x to FY20E consensus EPS of $0.655 and arrived at a target price offering an upside of lower double-digit (in percentage terms). Hence, we recommend a “Buy” rating on the stock at the current market price of $25.650, down 1.422% on 12th February 2020.
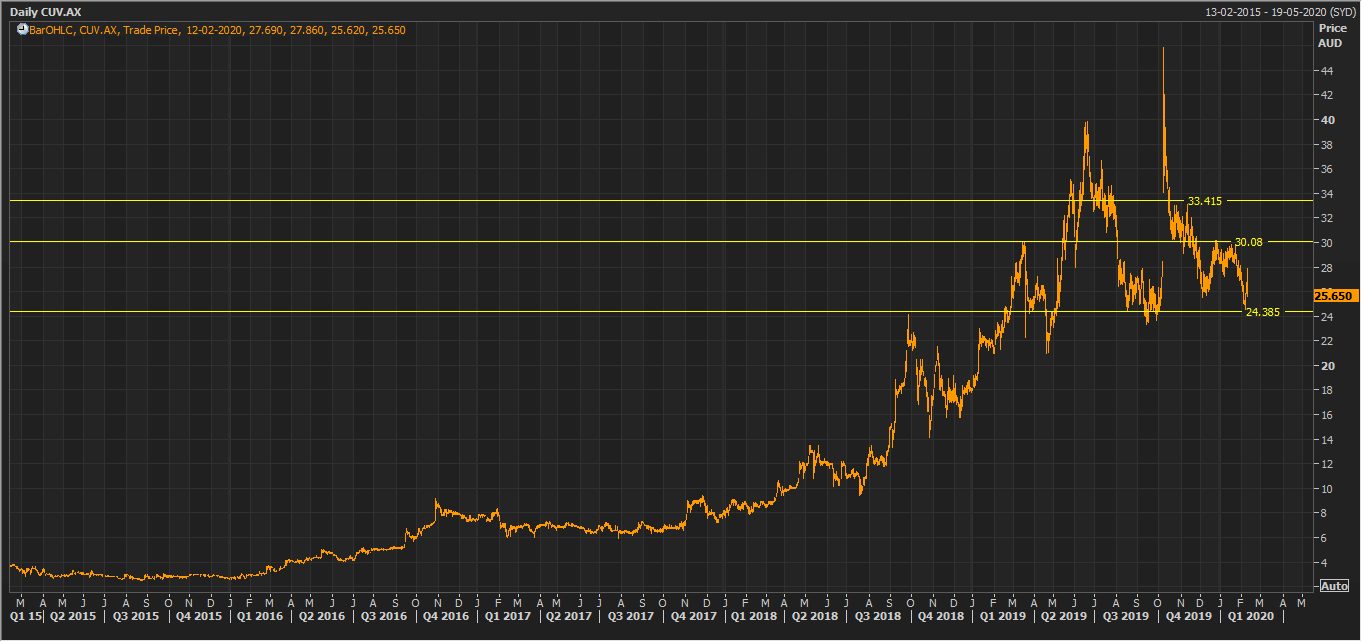
CUV Daily Technical Chart (Source: Thomson Reuters)
Disclaimer
The advice given by Kalkine Pty Ltd and provided on this website is general information only and it does not take into account your investment objectives, financial situation or needs. You should therefore consider whether the advice is appropriate to your investment objectives, financial situation and needs before acting upon it. You should seek advice from a financial adviser, stockbroker or other professional (including taxation and legal advice) as necessary before acting on any advice. Not all investments are appropriate for all people. Kalkine.com.au and associated pages are published by Kalkine Pty Ltd ABN 34 154 808 312 (Australian Financial Services License Number 425376). The information on this website has been prepared from a wide variety of sources, which Kalkine Pty Ltd, to the best of its knowledge and belief, considers accurate. You should make your own enquiries about any investments and we strongly suggest you seek advice before acting upon any recommendation. Kalkine Pty Ltd has made every effort to ensure the reliability of information contained in its newsletters and websites. All information represents our views at the date of publication and may change without notice. To the extent permitted by law, Kalkine Pty Ltd excludes all liability for any loss or damage arising from the use of this website and any information published (including any indirect or consequential loss, any data loss or data corruption). If the law prohibits this exclusion, Kalkine Pty Ltd hereby limits its liability, to the extent permitted by law to the resupply of services. There may be a product disclosure statement or other offer document for the securities and financial products we write about in Kalkine Reports. You should obtain a copy of the product disclosure statement or offer document before making any decision about whether to acquire the security or product. The link to our Terms & Conditions has been provided please go through them and also have a read of the Financial Services Guide. On the date of publishing this report (mentioned on the website), employees and/or associates of Kalkine Pty Ltd do not hold positions in any of the stocks covered on the website. These stocks can change any time and readers of the reports should not consider these stocks as personalised advice.
AU

.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)

 Please wait processing your request...
Please wait processing your request...