Lithium-Ion Battery
Updated on 2023-08-29T12:00:15.956639Z
A lithium-ion battery (LIB) is an umbrella term used for the group of rechargeable battery types in which the lithium ions migrate from the negative to the positive electrode during discharge and back while charging. Lithium-ion batteries have a high energy density with long-term charge retention and minimal loss, making it appropriate for varying applications including cell phones, laptops, modern robots, production equipment and cars.
Components of LIB:
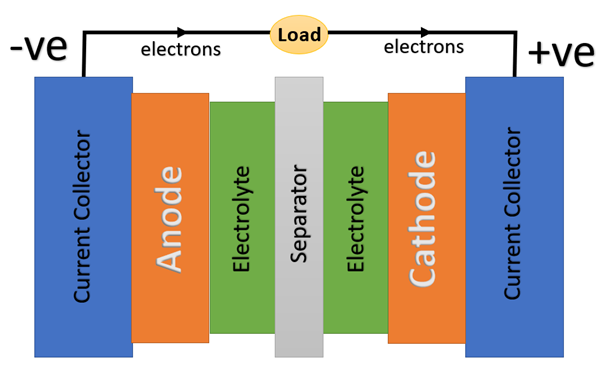
A typical LIB contains five main elements: electrolyte, separator, anode, cathode and two current collectors. The electrolyte with high ionic conductivity carries the positively charged lithium ions from the anode to the cathode and vice versa through the separator.
Kalkine Group Image
The electrolyte allows only ions to pass through it. The charge is produced at the positive current collector due to the movement of lithium ions, creating free electrons at the anode. The electrical current flows from the current collector through a device being powered to the negative current collector. The separator blocks the flow of electrons inside the battery while allowing small-sized lithium ions to move through the micro-permeable membrane.
History of LIB:
The work on lithium battery was started in 1912, after 95 years of lithium discovery (1817), under American physical chemist G.N. Lewis. Lithium is the lightest metal with the best electrochemical potential, giving the largest energy density for weight. Various attempts were made to develop rechargeable lithium batteries, but none could succeed due to safety problems arising from lithium metal instability. The problem in charging of lithium batteries pushed researchers to shift their interest towards lithium-ion driven non-metallic batteries. Although it provided slightly lower energy density but came with better safety as compared to lithium batteries. The production got commercialised in 1991 by Sony Corporation which was further followed by other companies too.
Lithium-Ion Versus Lead Acid Battery:
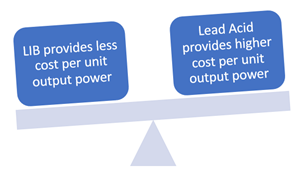
Cost:
Kalkine Group Image
Lead-acid is a well-known economical option accessible in enormous amounts with little concern regarding the stockpile's security. Lead-acid is an excellent fit for stationary applications where space is plentiful, and energy necessities are low.
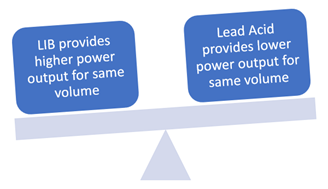
Power:
Kalkine Group Image
Lithium-ion is more efficient and compact as compared to lead-acid batteries. Lithium-ion batteries can achieve an energy density up to 125-600+ Wh/L; on the other hand, lead-acid batteries will reach up to 50-90 Wh/L of energy density. Lead-acid batteries require approximately ten times more space as compared to lithium-ion batteries, considering the same output range.
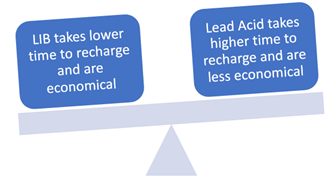
Recharging:
Kalkine Group Image
Lead-acid batteries may take up to 10 hours of charging time, depending on its size whereas lithium-ion batteries may take three hours or as little as minutes to charge. Also, LIBs allow faster current rate for quick charging.
Advantages of LIBs:
- LIBs provide high energy density, which is one of the most important advantages of lithium-ion batteries. Mobiles, electric cars, and other gadgets require a stable power supply for their usage.
- The self-discharge rate of LIBs is much lesser than that of many other rechargeable batteries. It comes around 5 per cent in the initial four hours after charging and further declines to 1-2 per cent per month after that.
- It has relatively low maintenance compared to other batteries that require periodic top-ups and periodic discharge for memory effects.
- Cell voltage offered by LIBs is around 3.0 V which is much higher than standard alkaline cells and lead-acid batteries, which provide 1.5 V and 2.0 V respectively.
- LIBs don’t require any priming before first time use use and are ready to go.
- LIBs are readily available in different types and forms, making them convenient to use in wide applications ranging from small cell phones to giant mega cars.
Disadvantages of LIBs:
- LIBs can withstand over 500-1000 charge and discharge cycles, after which their capacity falls.
- LIBs require protection from being under and overcharged as they need safe limits of charging for effective operations.
- Transportation of LIBs is limited to waterways as airways often ban them.
- LIBs are around forty per cent costlier than nickel-cadmium batteries.
- LIB is an evolving technology with high scope of further development. In recent years, frequent advancement is seen in LIBs, which can make this technology more efficient in the future.