Hydrogen fuel
Updated on 2023-08-29T12:00:51.156722Z
Hydrogen is the first element on the periodic table and also the lightest among all the elements found in nature. The combustion of hydrogen produces heat and water. Since there is no emission of any type of harmful gases, it is considered one of the cleanest fuels for energy production.
There is an ongoing discussion on the source and method of hydrogen generation, but hydrogen as fuel can be considered as an alternative to fossil fuels. Hydrogen can be conveniently used as a replacement for natural gas and liquid petroleum products. Hydrogen could be filled in fuel tanks of automobiles under high pressure and can work fine just as CNG with no carbon emission.
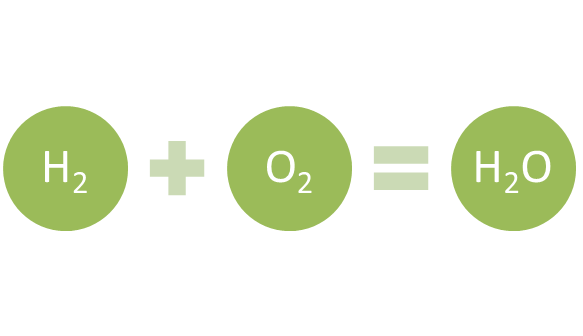
Hydrogen on combustion produces water and energy (Copyright © 2021 Kalkine Media)
Hydrogen could also be used as an alternative for LPG as a household fuel supply. There has been considerable work going on to improve the fuel cells performances running on hydrogen. The energy produced by 1 kilogram of hydrogen is equivalent to the energy produced by 2.8 kilograms of gasoline. Like gasoline, hydrogen does not emit harmful effluents and carbon gases. The only byproduct is water.
How abundant is hydrogen?
Hydrogen is the basic constituent of water and is usually present as atoms and molecules in fossil fuels. The chemical property of hydrogen to form covalent and electrovalent bonds with other atoms makes its presence abundant in nature.
Hydrogen can be extracted from water and hydrocarbons easily on a commercial scale. Water can be broken into its constituent molecules of hydrogen and oxygen using the electrolysis method. The method involves the supply of electricity in a significant amount, which may be generated using fossil fuels. The method is not considered efficient when it comes to producing clean fuel.
From hydrocarbons, catalysts are used to produce hydrogen, and in the process, carbon is also produced. Since hydrogen is gaining ground globally as an alternative fuel for reducing carbon emissions, new methods are being developed which are energy efficient and produce very less or no carbon.
What are the methods used for hydrogen production?
Several methods produce hydrogen on a large scale that can be classified into three main categories- thermochemical, electrolytic and biological process.
As the name suggests, the thermochemical method employs heat and chemical reactions to produce hydrogen. The basic principle of this method is to use thermal energy in combination with chemical cycles to break the hydrogen molecules from the molecular structure of the feedstock molecules.
Some of the existing thermochemical methods employed by the industry are natural gas reforming, coal gasification, biomass gasification, solar thermochemical hydrogen (STCH) and biomass-derived liquid reforming.
In the electrolytic method, electricity is passed at high voltage to break the water into oxygen and hydrogen. The whole apparatus is set in such a fashion that both the gases produced are stored in separate chambers. The method is not considered viable from the cleaner fuel point of view as the source of electricity may be fossil fuels.
Many solar and wind power projects are being set to produce electricity to power the electrolysis process to minimise their carbon footprint. The electrolysis method is fairly simple and is one of the widest use methods to produce hydrogen. The biological method uses certain types of microbes such as bacteria and algae to produce hydrogen through biological reactions. Sunlight and organic matter are used in the process to trigger the process, and being in the early stages of development has shown the potential of a possible sustainable method for producing hydrogen.
Microalgae or cyanobacteria use sunlight to break water into its constituent molecules -oxygen and hydrogen. The production rate is low and work is going on to increase the rate. In some case, organic matter is used which are digested by the microbes to produce hydrogen under the presence of sunlight.
Summary
- Hydrogen is gaining ground as an alternative clean fuel to curb carbon emission and stall the greenhouse effect.
- Commercially, hydrogen can be produced through several methods, including electrolytic to thermochemical methods.
- Biological methods which involve sunlight, organic matter and microbes are seen as a sustainable source of hydrogen production.
- NASA uses hydrogen as a fuel for their rockets shows the credibility of hydrogen as a fuel.
What are the main differences between ‘green’ and ‘blue’ hydrogen?
The hydrogen produced without using fossil fuel and zero carbon emission is called ‘green hydrogen’, while hydrogen produced using fossil fuel at any stage of the production is known as ‘blue’ hydrogen’. Hydrogen produced from coal and petroleum or from the electrolytic method using electricity sourced from power plants run on fossil fuels will come under the blue hydrogen category.
Copyright © 2021 Kalkine Media
Hydrogen produced using solar, wind and other forms of renewable energy sources in the electrolytic method can be called green hydrogen.
Is hydrogen safe as fuel from a safety point of view?
All fuels come with some degree of risks associated with them. EVs running on lithium battery are prone to fire hazard if not handled carefully. Gasoline and diesel are highly combustible and poses a risk. Natural gas is stored at high pressure, and damage to the container can cause catastrophic accidents. These all accidents and incidents have already occurred, and researchers and design engineers are working tirelessly to ensure more safety to these systems.
Hydrogen is the lightest element in nature and is also highly combustible. Hindenburg disaster is one such early example of accidents associated with hydrogen gas. But we also know that NASA uses hydrogen for their rocket fuels and have high safety records.
As a fuel, hydrogen can be stored in automobile tanks under high pressure of 500-1000 psi. Any leak in the tank, hydrogen would easily assimilate with the atmosphere without risk of causing any harm to the environment. Though they are combustible, they would catch fire only when they are brought in contact with flame or spark. The same is the case with natural gas.