Priority Review Designation
Updated on 2023-08-29T11:59:47.365531Z
What is a Priority Review Designation?
Before approval, each drug or therapy marketed in the US must proceed through a detailed review process of the Food and Drug Administration (FDA).
The FDA has four processes for making drugs that treat serious diseases available more rapidly- Fast Track Process, Breakthrough Therapy Designation, Accelerated Approval and Priority Review.
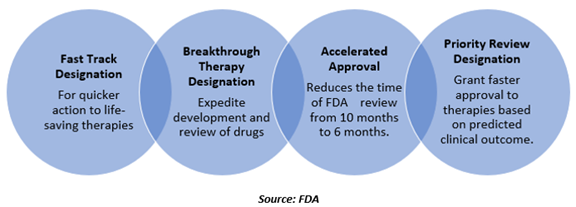
These programs accelerate as well as expedite development and review of therapies used to treat severe or life-threatening indications.
To read the drug development process, click here.
In 1992, under the PDUFA, the FDA agreed to some particular objectives for enhancing the time of review of a drug and formed a two-tiered system of review times Standard Review and Priority Review.
A Priority Review designation means the goal of FDA is to act on an application within six months as compared to ten months process under standard review.

The below-mentioned examples may demonstrate considerable improvement:
- Evidence of increased effectiveness in diagnosis, prevention, or treatment of a condition.
- Elimination or substantial decline of a treatment restricting drug reaction.
- Documented enhancement of patient compliance that is anticipated to lead to an improvement in serious outcomes.
- Evidence for safety as well as effectiveness in a new subpopulation.
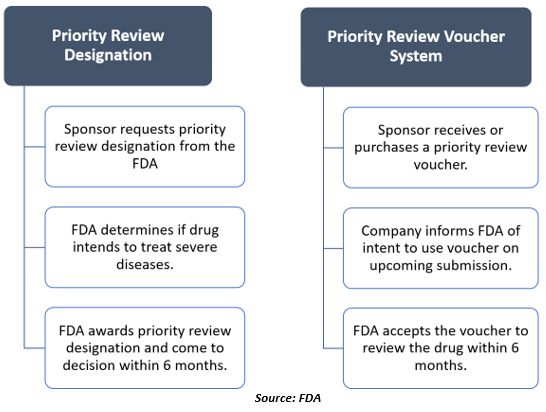
For every application, the FDA decides on the review designation. However, the sponsor or drug manufacturer may expressly request priority review as described in the Guidance for Industry Expedited Programs for Serious Conditions Drugs and Biologics.
Notably, it does not have any effect on the duration of the clinical trial. The agency advises the sponsor of a Priority Review designation within sixty days of the receipt of the original NDA, BLA, or efficacy supplement.
What are the qualifying criteria for Priority Review designation?
The criteria for a priority review designation are-
- For obtaining a grant of a priority review designation, the application must be for therapy or drug that is for treatment of severe disease and if approved, would offer a considerable improvement in safety along with efficacy.
- Any supplement that proposes a change in labeling according to a report on a pediatric study.
- An application for a treatment that has been designated as a qualified product for the treatment of infections.
- Any application for a drug proposed with a priority review voucher.
What is the process for Priority Review Designation?
The applicant may request priority review designation in the following three sections-

Sponsors can request priority review designation when they submit an original biologics license application (BLA), new drug application (NDA), or efficacy supplement.
How long does FDA priority review take?
A Priority Review designation indicates that the FDA will act on an application within six months as compared to ten months under the standard review process. This is a significant improvement for the safety or efficacy of the diagnosis, prevention, or treatment of any severe or life-threatening conditions as compared to standard applications.
What is Priority review voucher program?
The priority review voucher (PRV) program grants a voucher for priority review to a sponsor or drug manufacturer as an incentive for manufacturing drugs that might otherwise not be profitable to develop because of a smaller group of patients requiring treatment or for orphan diseases.
For using a priority review voucher, a sponsor must inform the FDA of their intention 90 days before its submission.
The FDA is also responsible for implementing three priority review voucher programs, which are meant for promoting the development of treatment or therapies for tropical indication, rare pediatric indications along with some medical countermeasures.
Once the FDA gives a priority review voucher to the drug manufacturing company or sponsor, the manufacturer can redeem the voucher by submitting a drug application for a drug in the future. The voucher will shorten the targeted review time for the drug by the FDA from 10 months to six months.
This reduction in the review period can be made even if the treatment in that future application would not be eligible for priority review on its own merits. The drug manufacturer also has an option to sell or transfer the voucher to another drug developer, which could then decide to use it or can similarly sell or transfer it to other manufacturers.
Recent Examples of Drugs Receiving Priority Review Designation
Bristol Myers Squibb and bluebird bio receive Priority Review Designation for multiple myeloma treatment
New York-headquartered Bristol Myers Squibb and bluebird bio announced on 22 September 2020 that the FDA accepted their Biologics License Application for Priority Review. The BLA was for idecabtagene vicleucel, the two partners’ Anti-BCMA CAR T cell therapy. The PDUFA date is set for 27 March 2021.
US FDA accepts Orphazyme’s arimoclomol for Priority Review
Denmark-headquartered biopharma player Orphazyme received Priority Review Designation from the FDA for arimoclomol, the company’s treatment for NPC (Niemann-Pick disease type C), a rare genetic disorder. The designation was received on 16 September 2020, and the PDUFA action date by the FDA is set at 17 March 2021. The reduced review period will help the Company launch the product quickly if approved.